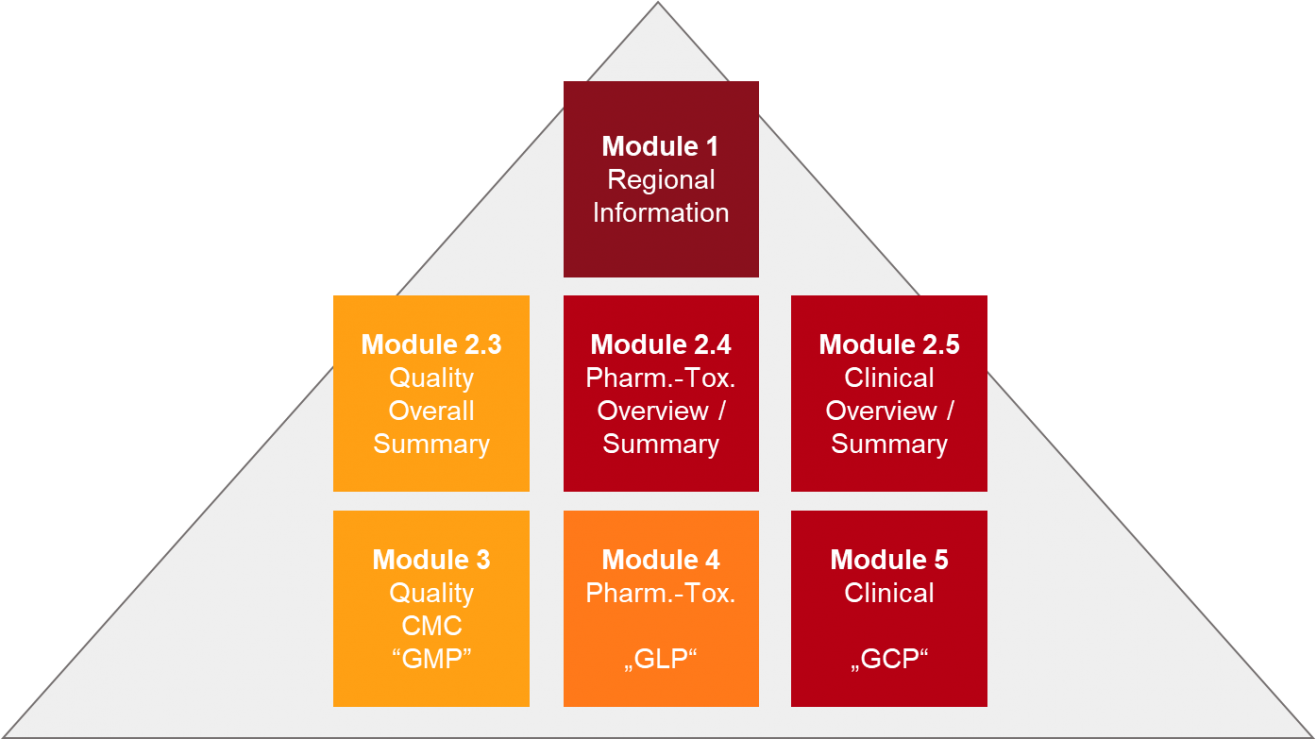
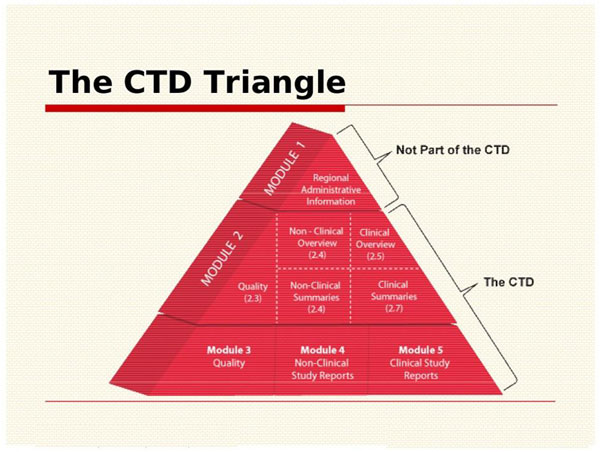
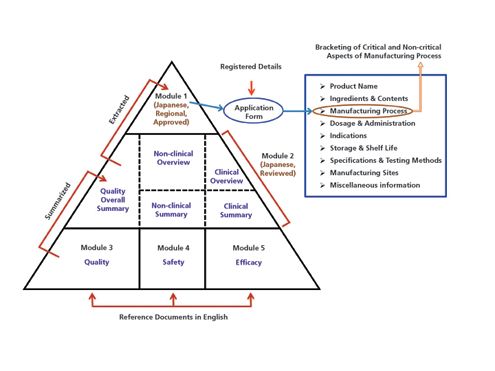
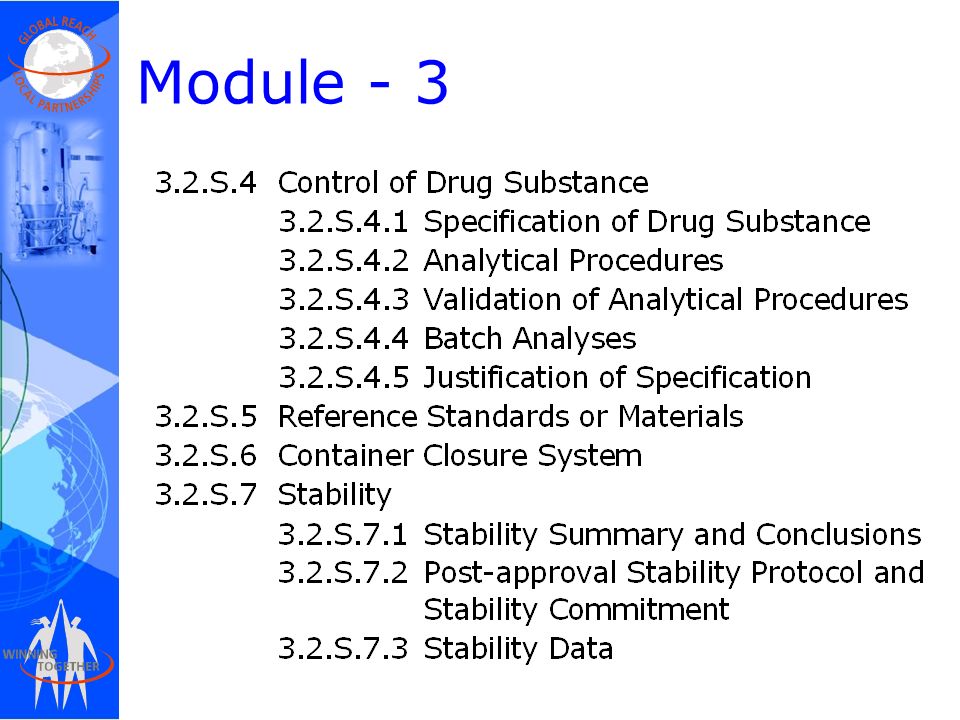
Drug substances in the drug product dossier - - Quality documentation requirements for marketing authorizations of medicinal products in Europe

ICH CTD QUALITY Part -CMC Module 3 Drug Substance Video by Rajashri Ojha at Raaj PharmaeLearning - YouTube
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan
WHO Guidelines on submission of documentation for the procedure for prequalification of similar biotherapeutic products