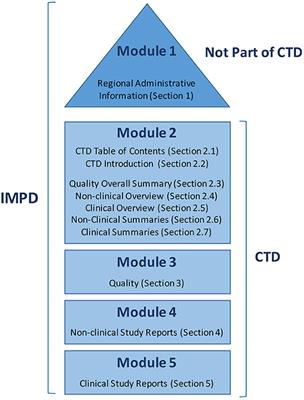
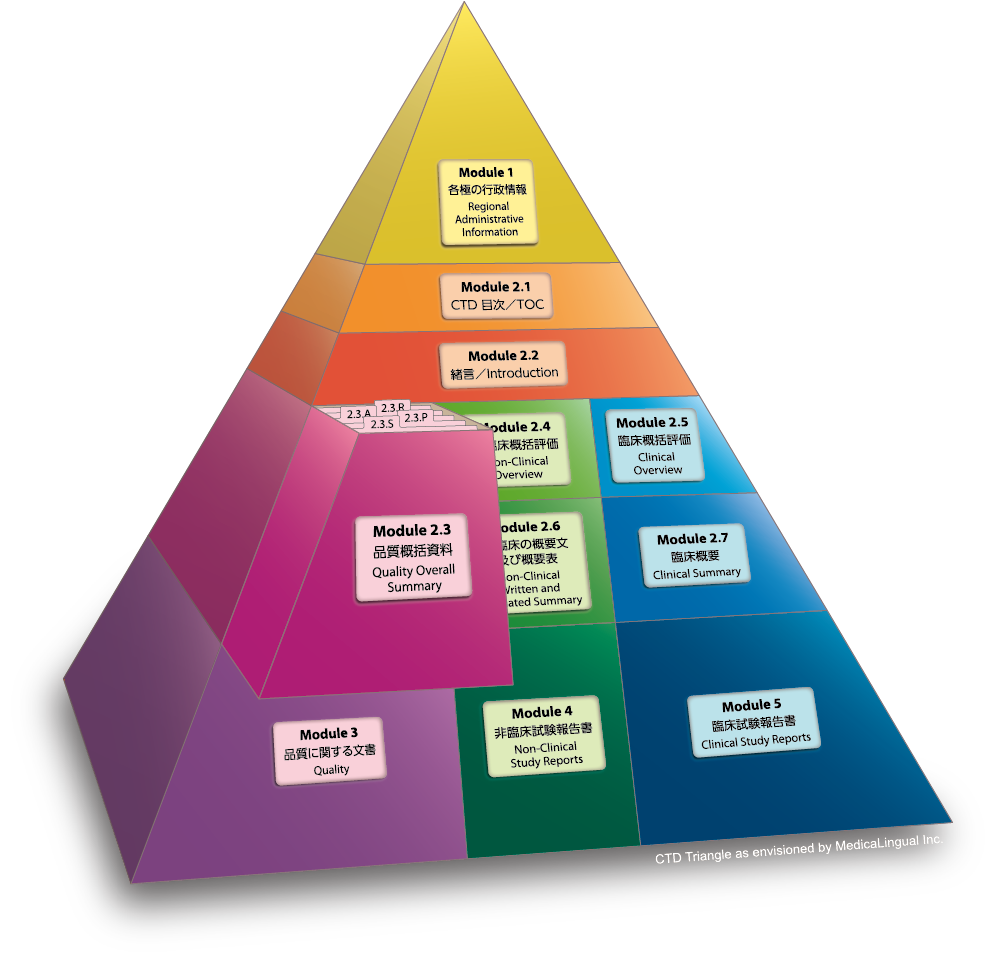
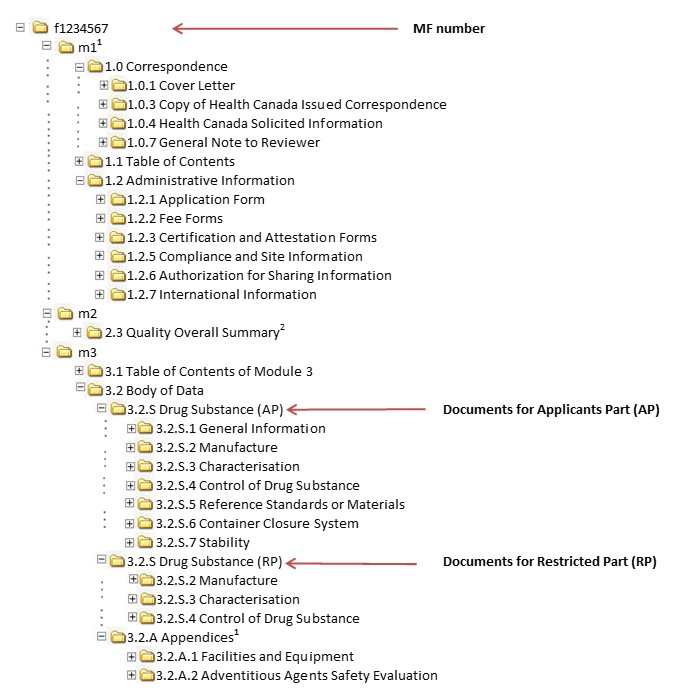
ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub

FDA Reviewer Reveals Tips on QbR for Drug Substance – Quality by Design for Biotech, Pharmaceutical and Medical Devices
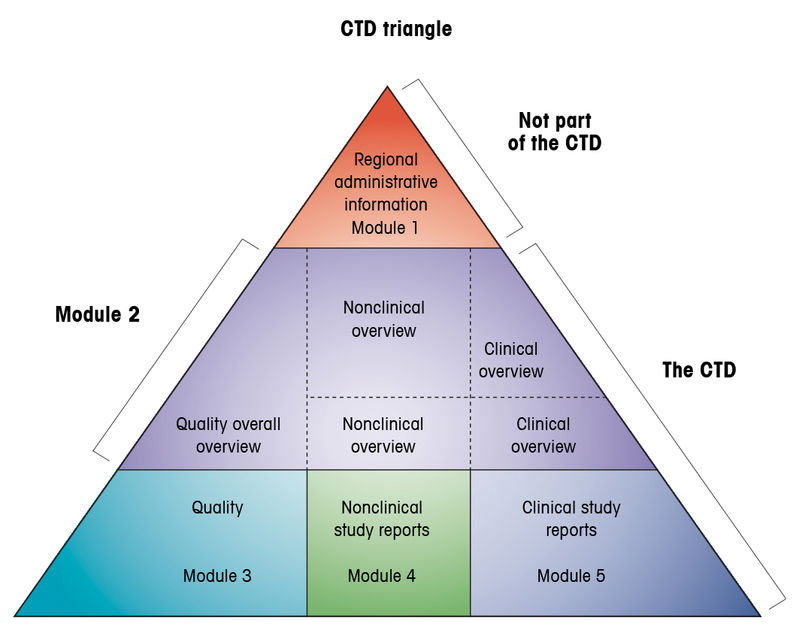
Drug substances in the drug product dossier - - Quality documentation requirements for marketing authorizations of medicinal products in Europe
WHO Guidelines on submission of documentation for the pilot procedure for prequalification of similar biotherapeutic products fo

Ivowen Ltd1 Ivowen Limited Preparation and Submission of a Traditional Herbal Medicinal Product Application. - ppt download