COVID-19 Vaccine Boosters vs. Third Doses: Frequently Asked Questions - Anne Arundel County Department of Health

COVID-19 Vaccine Second-Dose Completion and Interval Between First and Second Doses Among Vaccinated Persons — United States, December 14, 2020−February 14, 2021 | MMWR

Hawaii State Department of Health - What vaccine options are available for 16 & 17 year olds? The Pfizer vaccine is currently the only vaccine authorized for people under age 18. The

COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study - The Lancet Infectious Diseases
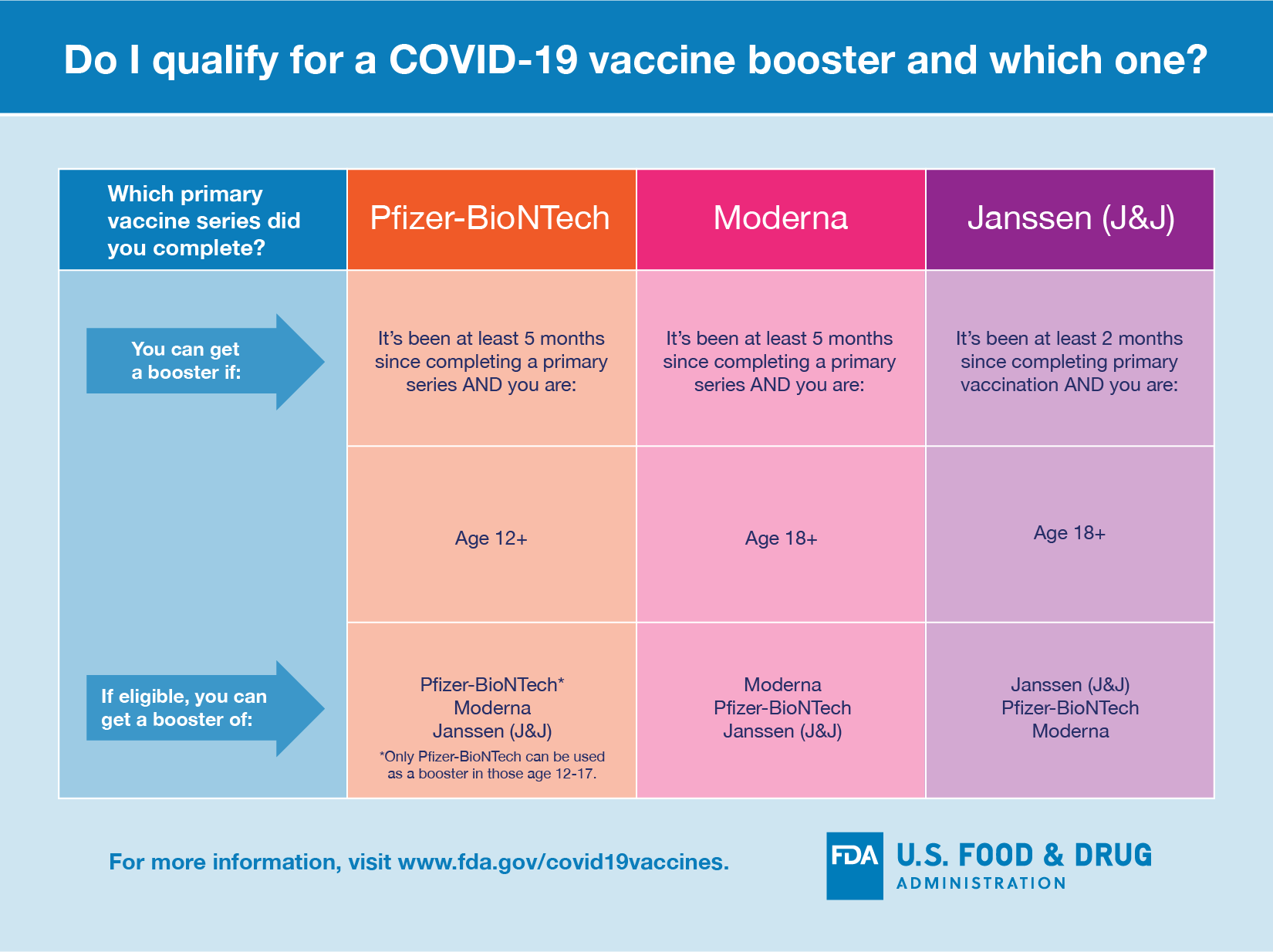
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA

Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study | The BMJ
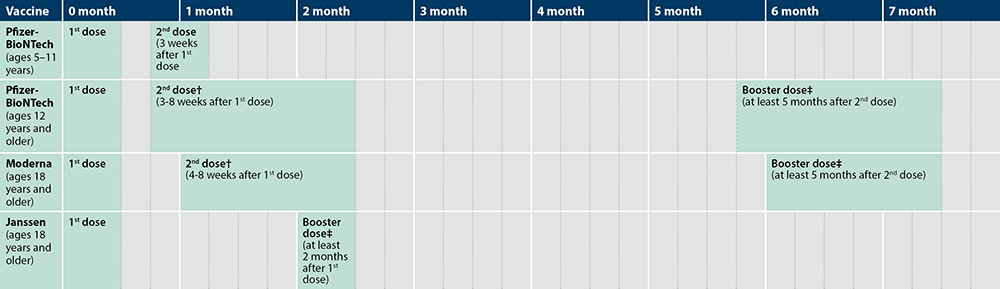
CDC updates COVID-19 vaccine recommended wait time to eight weeks for some groups | Children's Minnesota

Comparative effectiveness over time of the mRNA-1273 (Moderna) vaccine and the BNT162b2 (Pfizer-BioNTech) vaccine | Nature Communications

Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers — Eight U.S. Locations, December
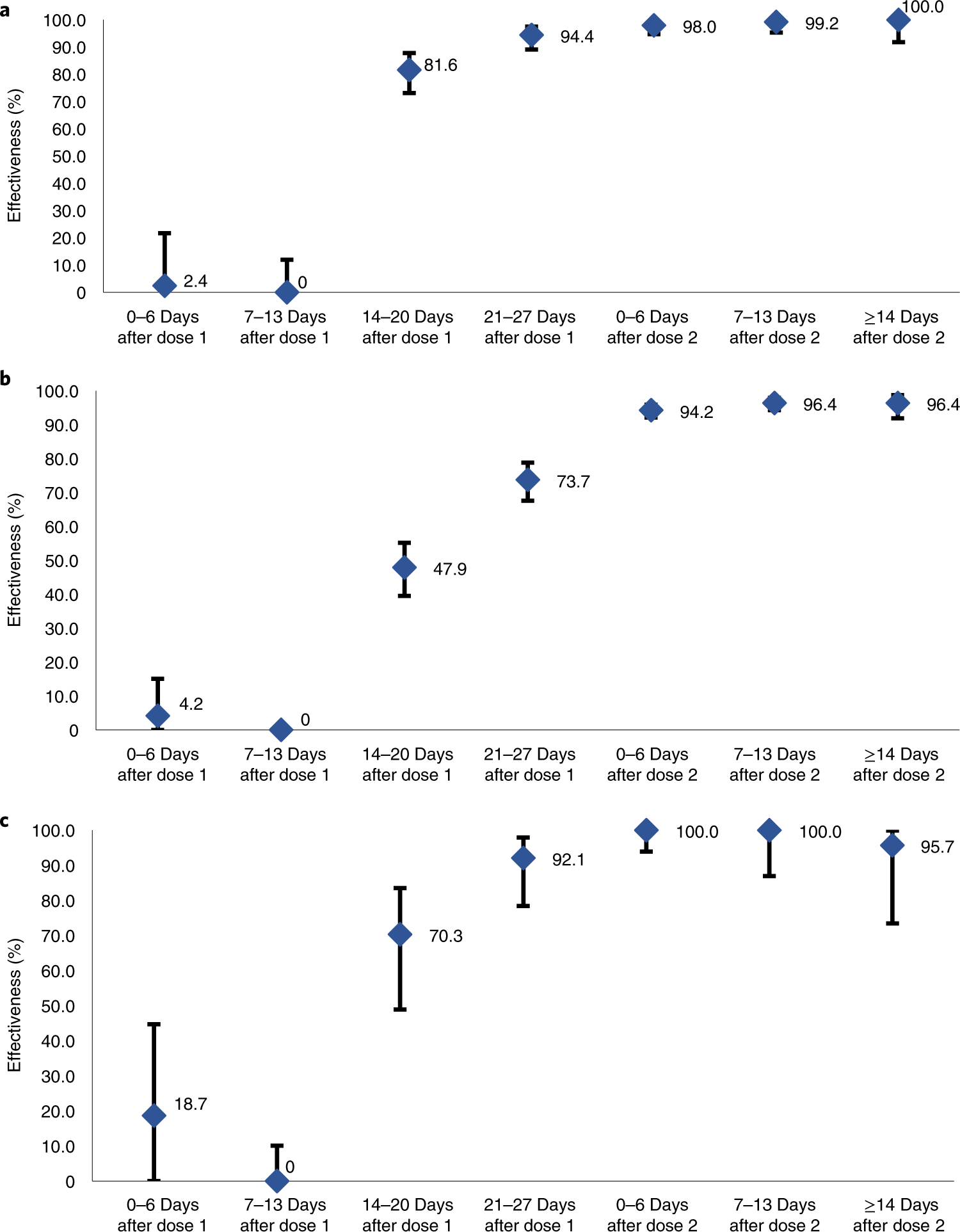
mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar | Nature Medicine
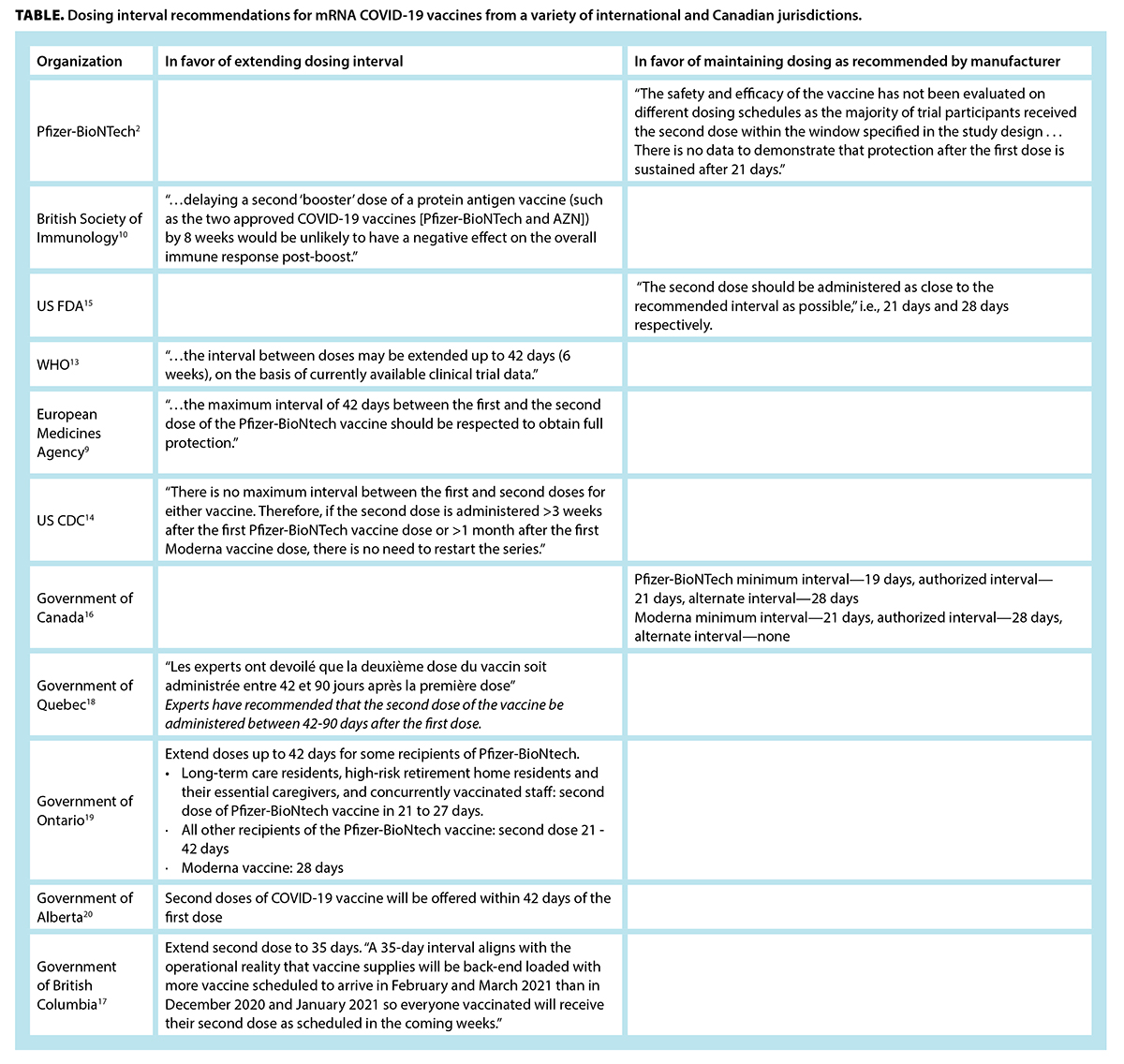
What is the evidence for extending the SARS-CoV-2 (COVID-19) vaccine dosing schedule? | British Columbia Medical Journal

Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study | The BMJ

ATAGI statement on use of the Moderna bivalent Original/Omicron vaccine | Australian Government Department of Health and Aged Care

Missouri Department of Health & Senior Services on Twitter: "You may have heard that you don't have to quarantine after you receive a COVID-19 vaccine. Here is a breakdown of timing for