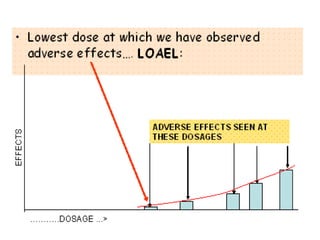
Workshop on the Guideline for First-In-Man Clinical Trials for Potential High-Risk Medicinal Products

The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies - ScienceDirect

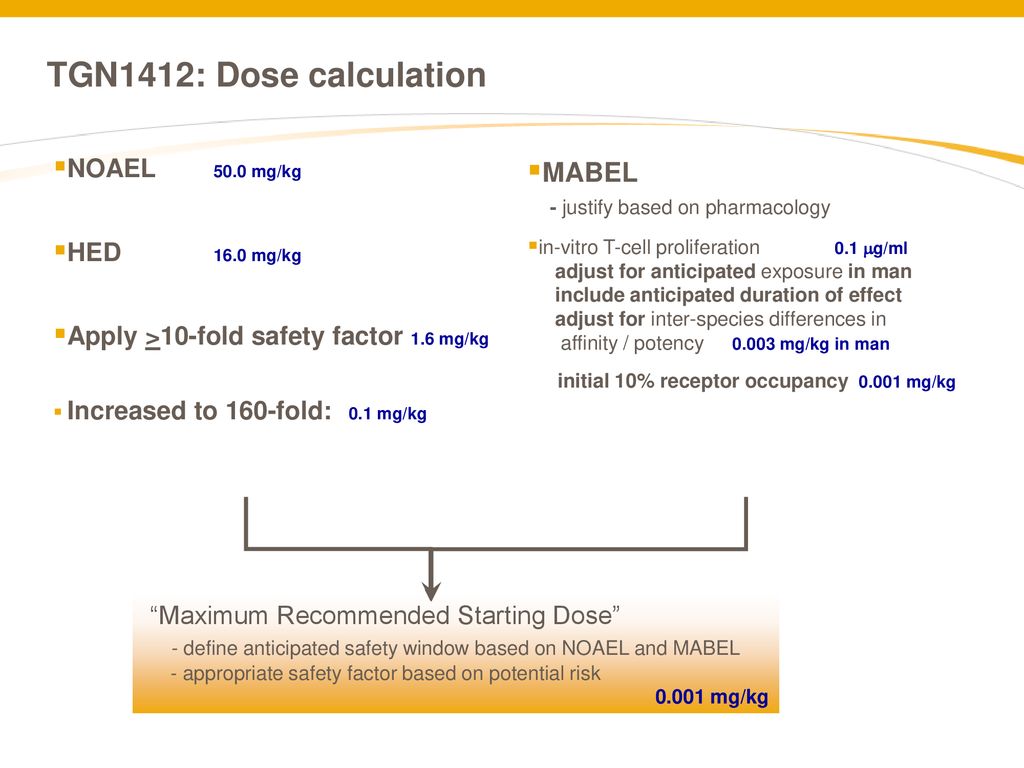
Calculation of the FIH dose from MABEL and NOAEL. The FIH dose from... | Download Scientific Diagram

Application of pharmacokinetics–pharmacodynamics/clinical response modeling and simulation for biologics drug development - Journal of Pharmaceutical Sciences

The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies - ScienceDirect

Calculation of the FIH dose from MABEL and NOAEL. The FIH dose from... | Download Scientific Diagram

The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies - ScienceDirect
Workshop on the Guideline for First-In-Man Clinical Trials for Potential High-Risk Medicinal Products