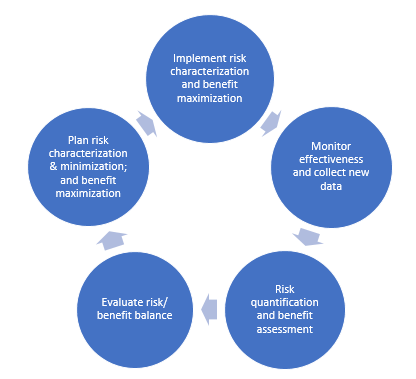
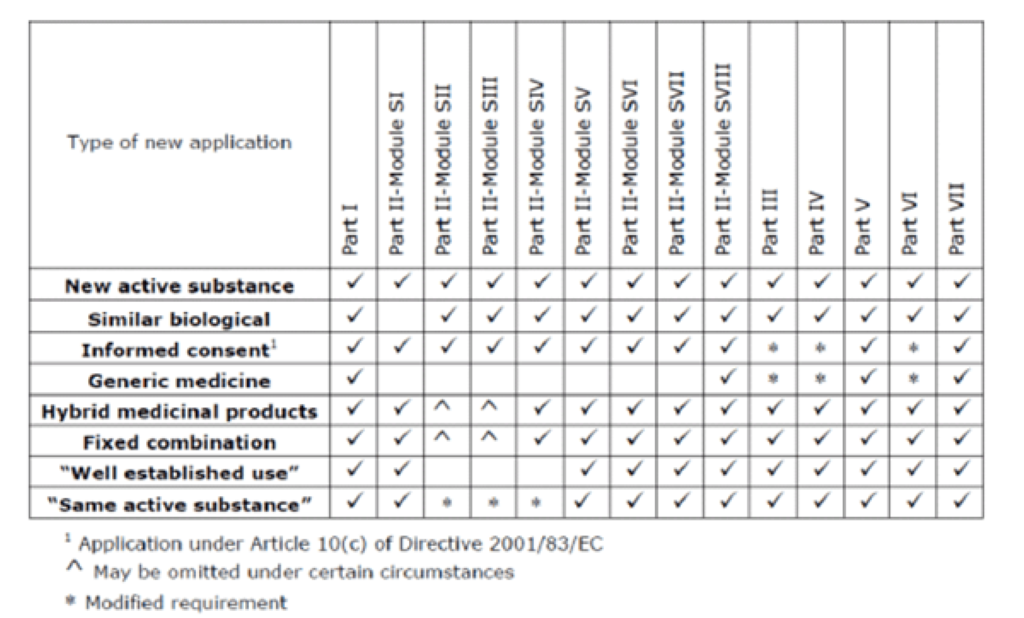
Impact of Changing Regulations and the Dynamic Nature of European Risk Management Plans for Human Medicines on the Lifecycle of Safety Concerns | SpringerLink
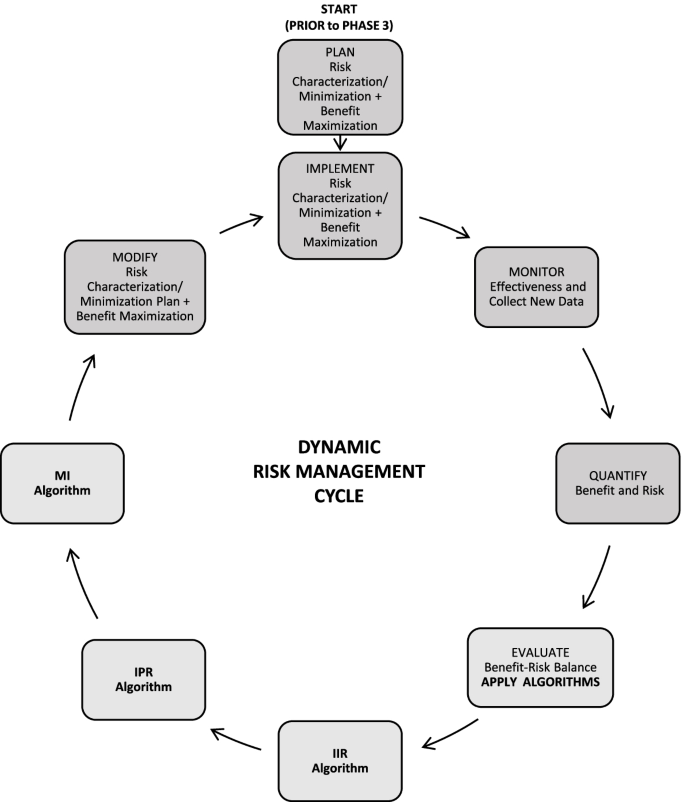
Risk Management Plans: reassessment of safety concerns based on Good Pharmacovigilance Practices Module V (Revision 2)—a company experience | Journal of Pharmaceutical Health Care and Sciences | Full Text