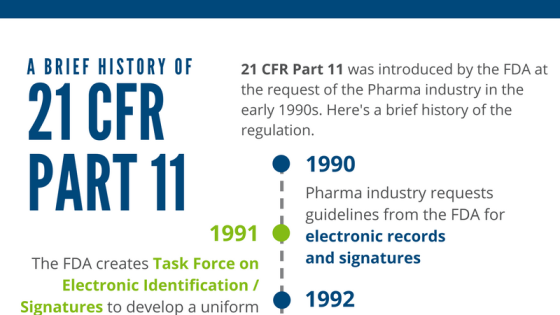
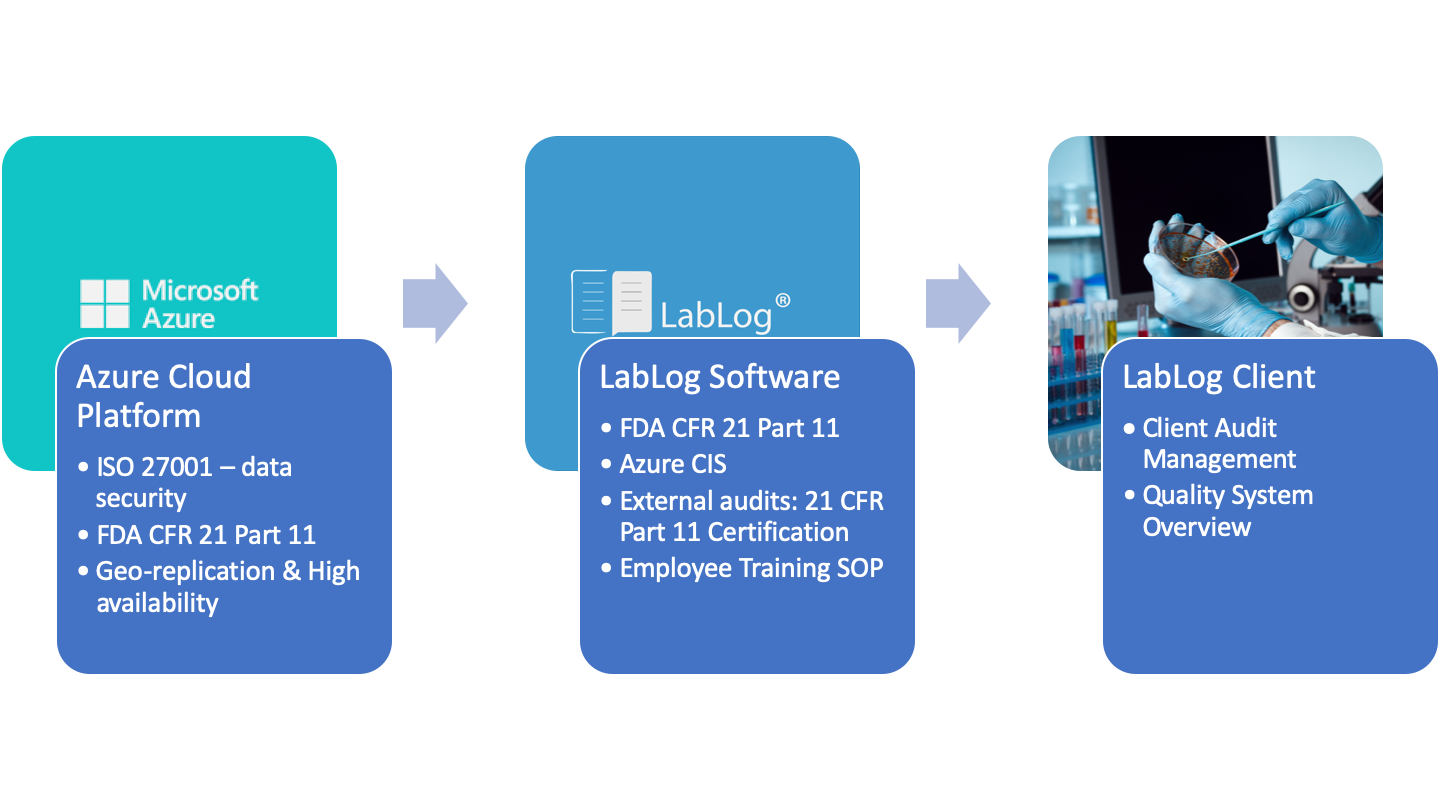
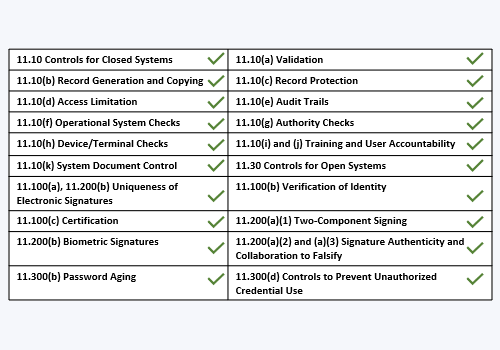
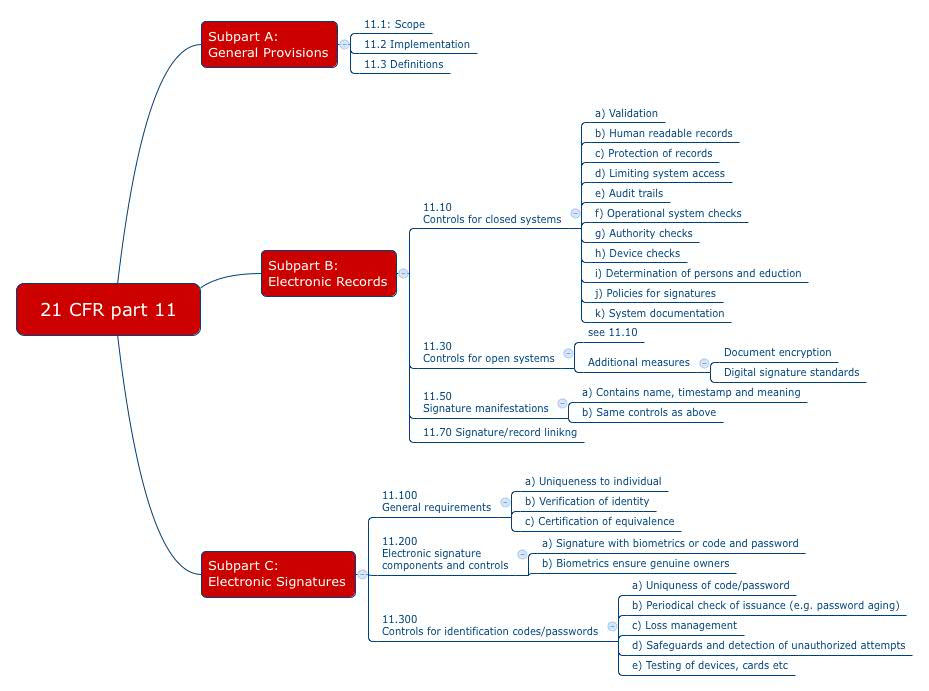
21 CFR Part 11, FDA's Guidance for Electronic Records and Electronic Signatures - Webinar (Recorded)

FDA 21 CFR Part 11 Electronic Records, Electronic Signatures, Scope and Application Testing Standard

ICT01: Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures | Zenosis – Learning for Life