Potential to Simplify the Writing of Submission Documents: Evaluation of Publicly Available Module 2 Documents in Drug Submissio

A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor
Exposé zum Thema: eCTD - Neue Wege der elektronischen Arzneimittelzulassung und die Vernetzung elektronischer regulatorischer P

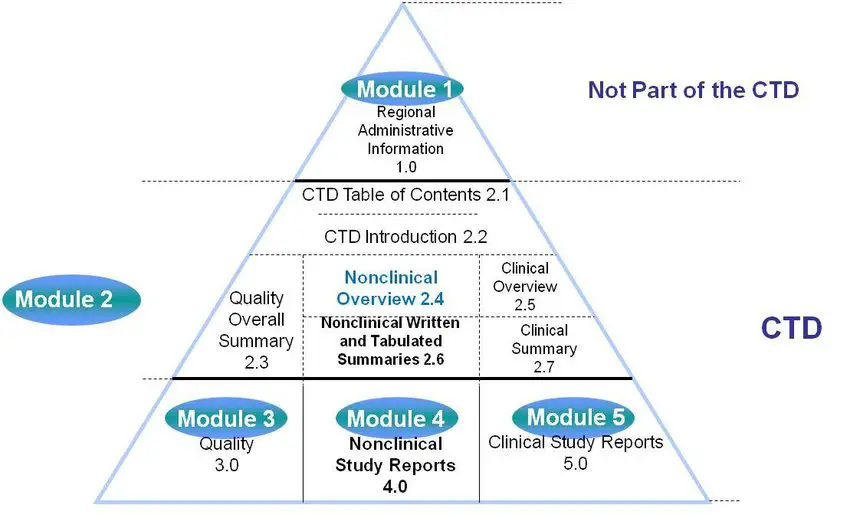
ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub
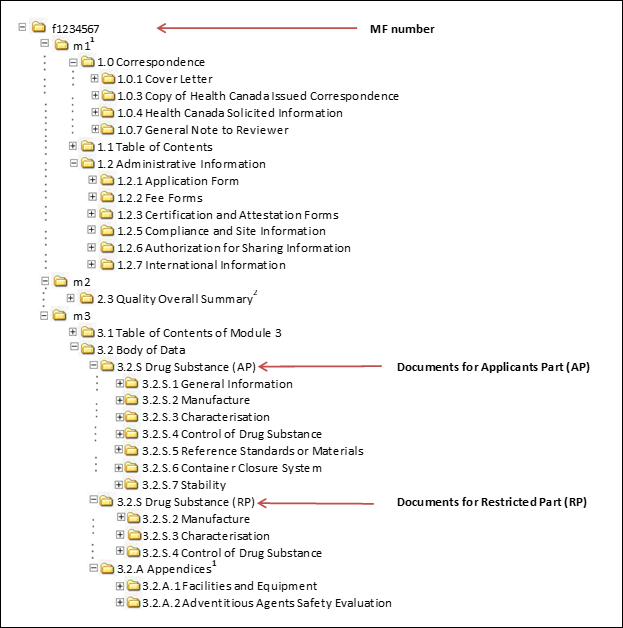
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format | Zenosis – Learning for Life
![PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/30903f1fc51c4917a2877b9cf3756ccc7fc6425a/8-Figure1-1.png)
PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar

Schematic representation of the five modules in the Common Technical... | Download Scientific Diagram