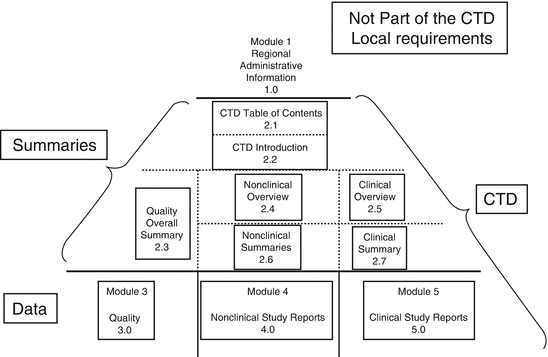
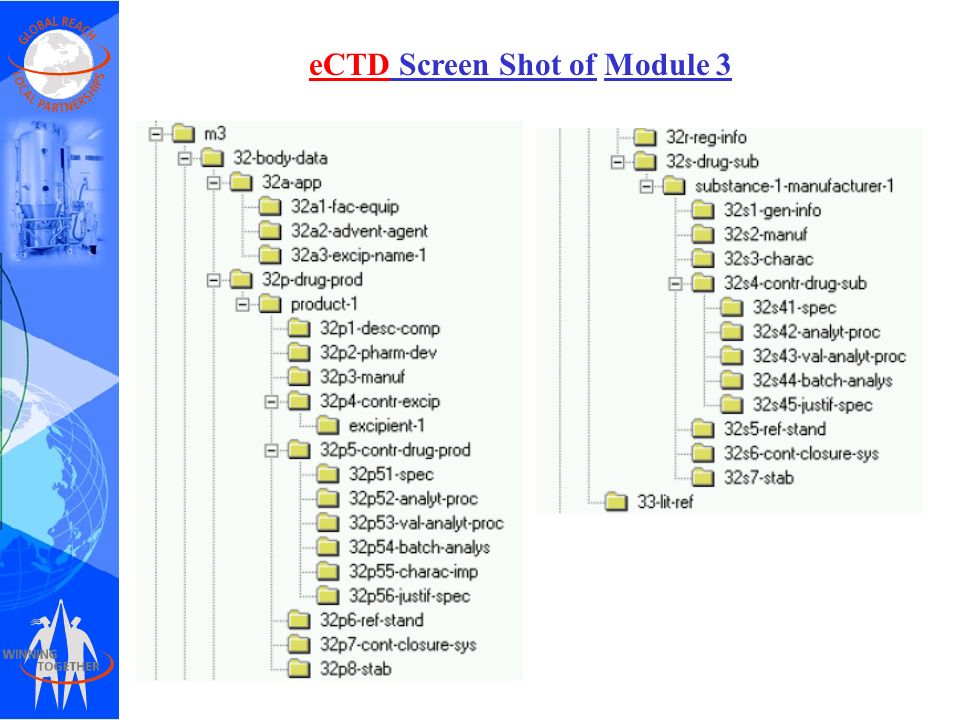
The Future of CMC Regulatory Submissions: Streamlining Activities Using Structured Content and Data Management - ScienceDirect
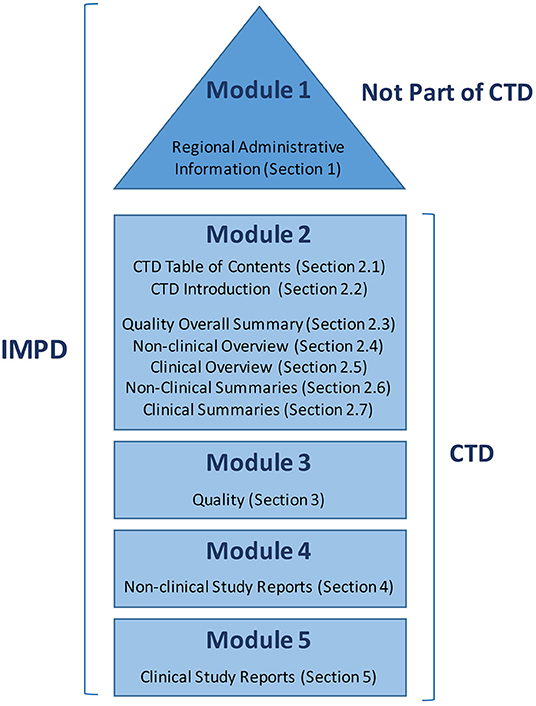
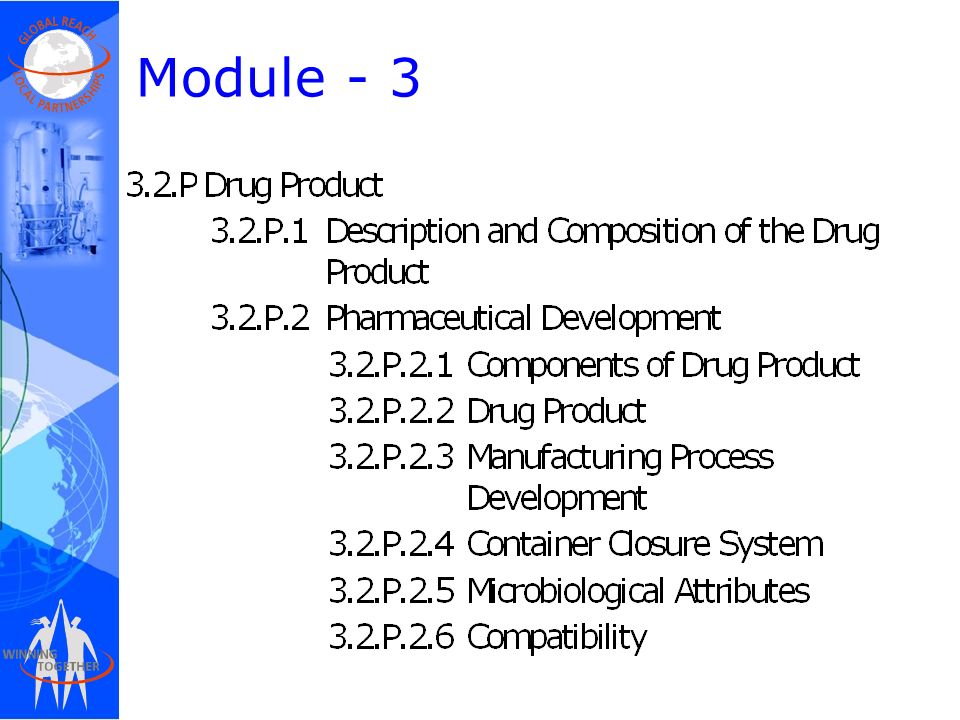
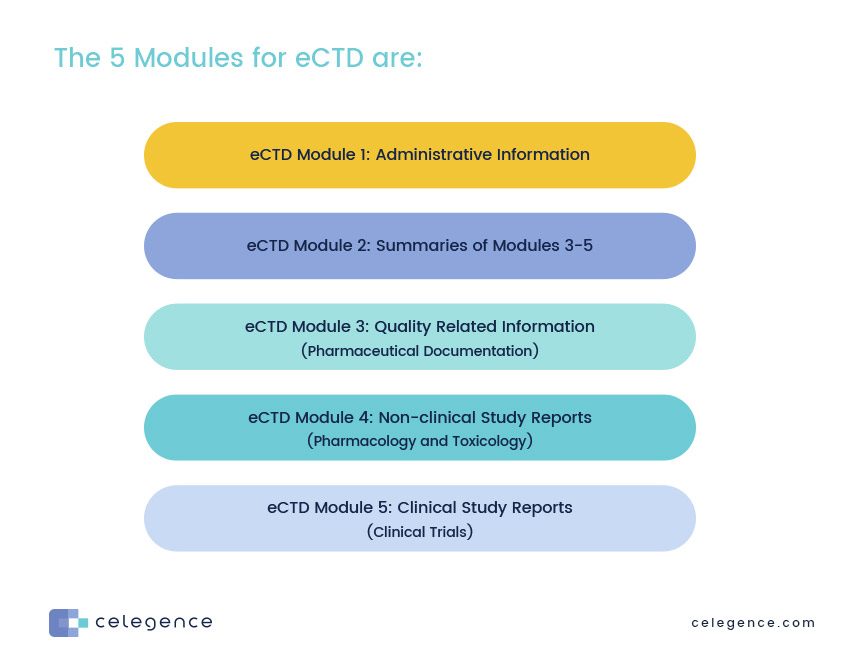
Guideline on the use of the CTD format in the preparation of a registration application for traditional herbal medicinal product