WHO Guidelines on submission of documentation for the pilot procedure for prequalification of similar biotherapeutic products fo

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

TuraSkills shares tip for writing #Module 2.7 #Section 2.7.2 #Summary of clinical pharmacology Studies #CS #Cl… | Pharmacology studying, Science blog, Writing tips
Preparing the Common Technical Document for Registration of Pharmaceuticals for Human Use (CTD)—Insights and Recommendations
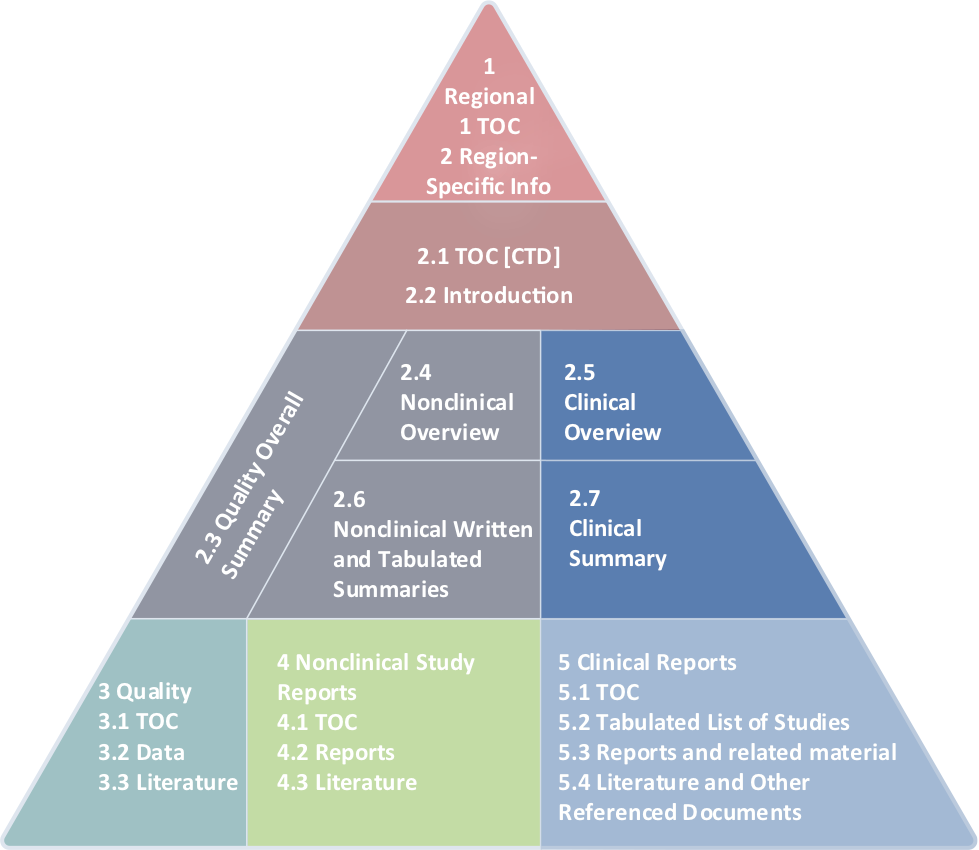
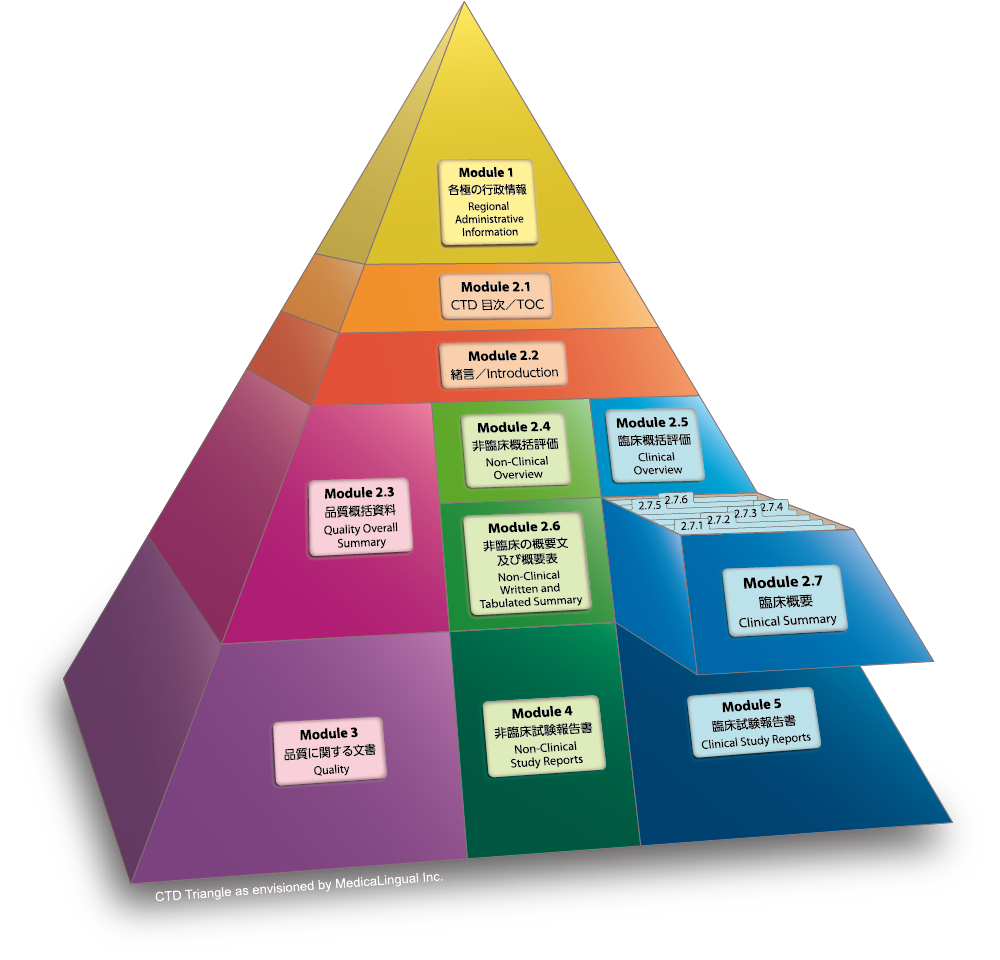
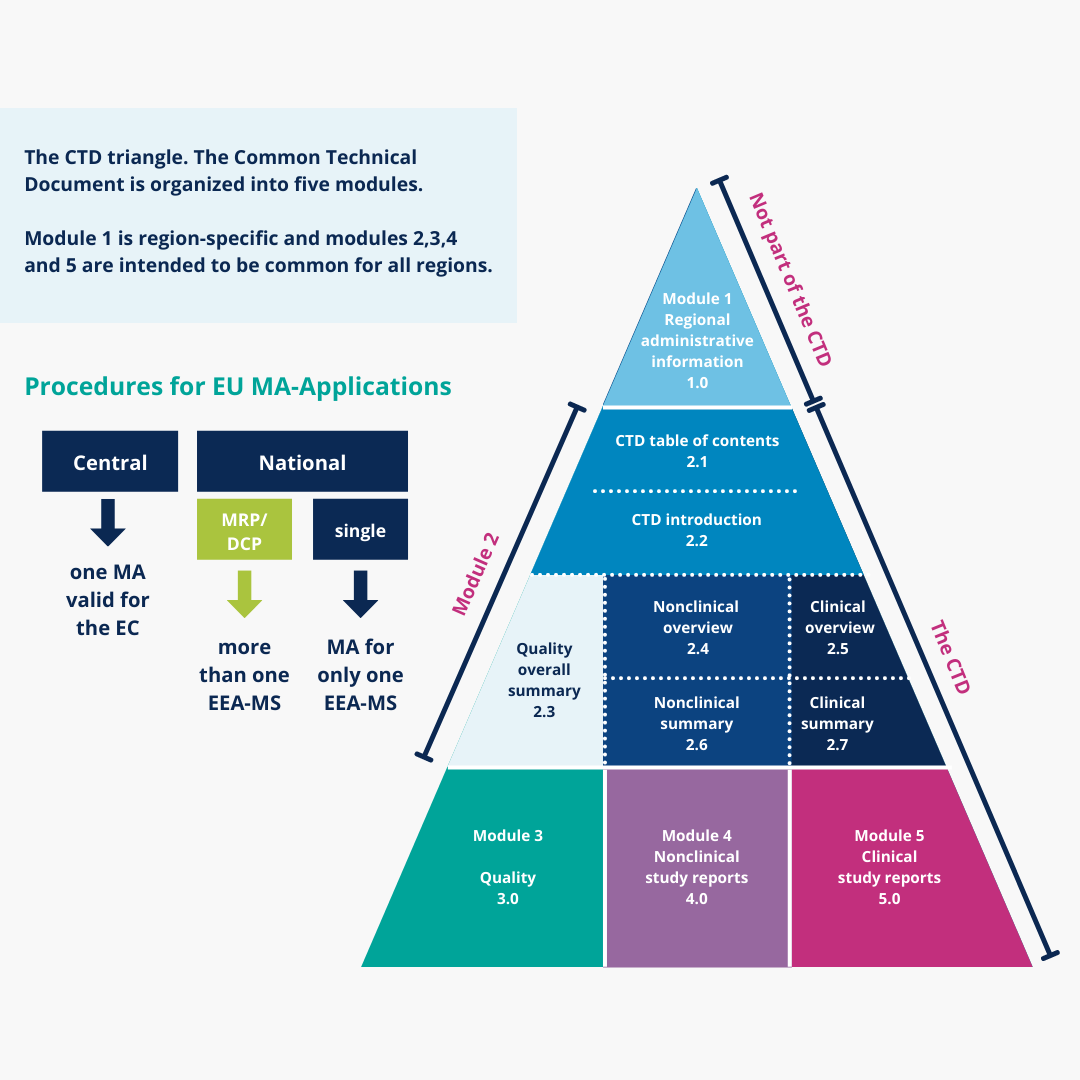
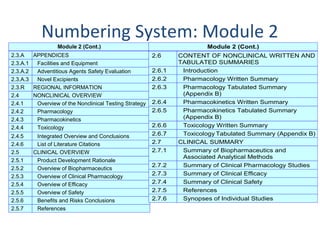
M4 Organization of the Common Technical Document for the Registration of Pharmaceuticals for Human Use Guidance for Industry
M4E(R2) - Common Technical Document for the Registration of Pharmaceuticals for Human Use - Efficacy

Representation of the components of the CTD. The nonclinical components... | Download Scientific Diagram
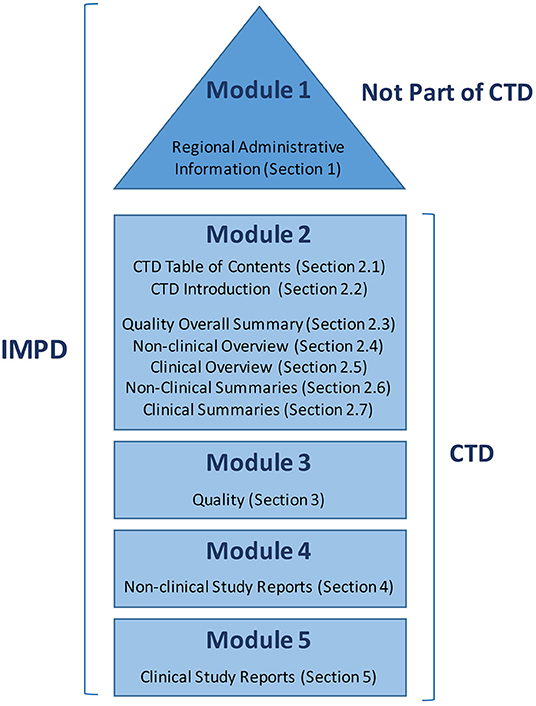
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience
Exposé zum Thema: eCTD - Neue Wege der elektronischen Arzneimittelzulassung und die Vernetzung elektronischer regulatorischer P

TuraSkills shares tip for writing #Module 2.7 #Sections of clinical summary #Clinical Summary #CTD Summary #Common Technical… | Study skills, Writing tips, Writing

An Introduction to Integrated Summary of Safety and Integrated Summary of Effectiveness (ISS and ISE) - Quantics Biostatistics
Potential to Simplify the Writing of Submission Documents: Evaluation of Publicly Available Module 2 Documents in Drug Submissio