Sanofi Genzyme Libtayo Cemiplimab 350Mg, Storage: 2-8 Degrees, Dosage Form: 1 Vial at Rs 400000/box in Delhi

Oral ulcers and sarcoid-like reaction in lymph nodes after cemiplimab therapy for locally advanced cutaneous squamous cell carcinoma: a case report - einstein (São Paulo)
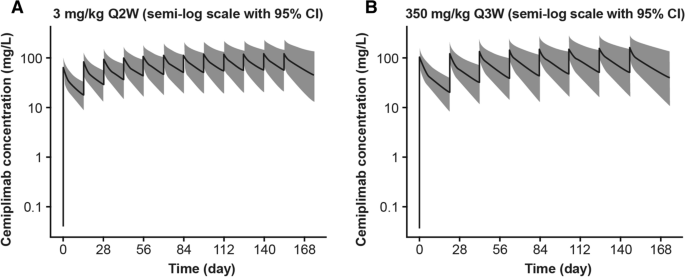
Fixed Dose of Cemiplimab in Patients with Advanced Malignancies Based on Population Pharmacokinetic Analysis | SpringerLink

Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial - The Lancet

PDF) Dose exploration results from Phase 1 study of cemiplimab, a human monoclonal programmed death (PD)-1 antibody, in Japanese patients with advanced malignancies
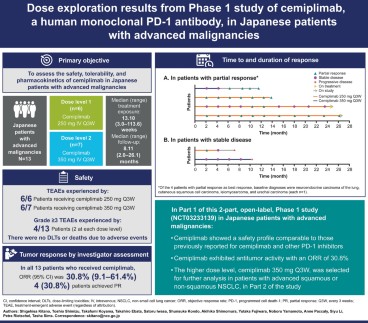
Dose exploration results from Phase 1 study of cemiplimab, a human monoclonal programmed death (PD)-1 antibody, in Japanese patients with advanced malignancies | springermedizin.de

Cancers | Free Full-Text | Real-Life Study of the Benefit of Concomitant Radiotherapy with Cemiplimab in Advanced Cutaneous Squamous Cell Carcinoma (cSCC): A Retrospective Cohort Study

Dose exploration results from Phase 1 study of cemiplimab, a human monoclonal programmed death (PD)-1 antibody, in Japanese patients with advanced malignancies | springermedizin.de

EMPOWER-lung 4: Phase II, randomized, open-label high dose or standard dose cemiplimab alone/plus ipilimumab in the secondline treatment of advanced non-small cell lung cancer (NSCLC)
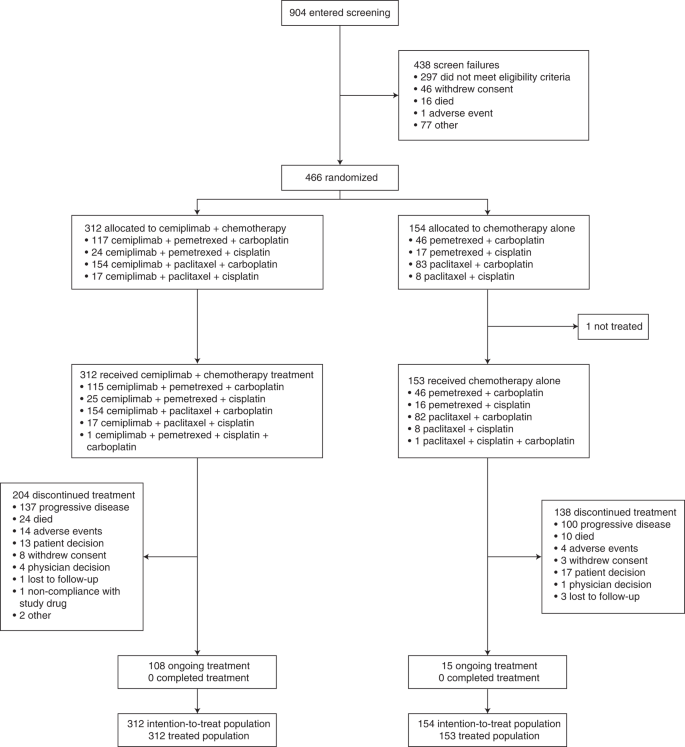
Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial | Nature Medicine

Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial - The Lancet Oncology

PDF) Fixed Dose of Cemiplimab in Patients with Advanced Malignancies Based on Population Pharmacokinetic Analysis

Dose exploration results from Phase 1 study of cemiplimab, a human monoclonal programmed death (PD)-1 antibody, in Japanese patients with advanced malignancies | springermedizin.de